|
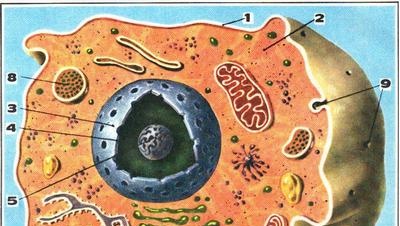
 Transuranic elements are the brainchild of modern technology. Laboratories with sophisticated equipment, nuclear reactors - these are the "deposits" from which, at the cost of huge energy expenditures, are now receiving insignificant quantities of elements that are not found in nature. Transuranic elements are the brainchild of modern technology. Laboratories with sophisticated equipment, nuclear reactors - these are the "deposits" from which, at the cost of huge energy expenditures, are now receiving insignificant quantities of elements that are not found in nature.
Hours, minutes, seconds, even fractions of a second - such is the duration of their existence. If they existed in the early periods of the geological history of the Earth, then for 5-6 billion years of the life of our planet they disappeared.
But at the end of the 40s, a transuranic element, plutonium, was discovered in nature. It turned out that according to all forecasts, this disappeared element is found in a number of uranium-thorium minerals. True, the plutonium content in them is very small - ten billionths of a gram per ton of rock. Yet it is determined both chemically and using precise methods of measuring radioactivity.
In nature, plutonium is created, obviously, in the same way as in atomic reactors: neutrons released during the decay of uranium nuclei, meeting on their way with other uranium-238 nuclei, are captured by them, and as a result, plutonium-239 nuclei appear. But under natural conditions, on the way of neutrons, they are found in a great variety of nuclei of foreign elements that make up a mineral or rock. These nuclei absorb neutrons and take them out of the game. That is why the "production" of natural "nuclear reactors" is so small.
However, plutonium isotopes live for thousands, tens of thousands, even tens of millions of years, and therefore they can accumulate. And the short life span of other transurans clearly gave no hope of meeting them in nature. It is not surprising that until very recently it was considered: plutonium is the last element of the periodic table, still found on our planet.
But the research of a group of Soviet physicists and chemists headed by V.V.Cherdyntsev refuted this long-standing opinion.
More than once, cases were noted when the sample under study turned out to be more radioactive than one would have expected, judging by the amount of radioactive elements and intermediate decay products contained in it.
For a long time, no explanation could be found for this phenomenon. After the discovery of plutonium in uranium ores, it was found that in most cases it is its presence that causes excessive activity. Since then, it has been assumed that whenever a sample is found to be more active than it should be, the excess should be attributed to plutonium.
However, VV Cherdyntsev's group, conducting a study of the isotopic composition of radioactive minerals, found that in a number of cases the sum of the activity of all radioactive elements, even with the addition of plutonium and radioactive intermediate products of its decay, is still less than the actually observed activity. The researchers, naturally, had the assumption that they should look for some other radioactive element, which cannot be chemically captured.
 The study of strange samples has shown that they have an excess of uranium-235 compared to the theoretically calculated amount. But uranium-235 is the final decay product of the sauranium element curium obtained in the laboratory. If this is so, then in nature there is not a short-lived laboratory curium, but some of its long-lived isotope. The study of strange samples has shown that they have an excess of uranium-235 compared to the theoretically calculated amount. But uranium-235 is the final decay product of the sauranium element curium obtained in the laboratory. If this is so, then in nature there is not a short-lived laboratory curium, but some of its long-lived isotope.
It was decided to try to find him.
Endless measurements ... And here's the result: a long-lived isotope, curium-247, has been discovered, with a half-life of approximately 250 million years. Therefore, there is another sauranium element in nature!
But among the intermediate decay products of curium should be americium-243. So, therefore, americium should also be found in nature.A new series of measurements - and the assumption is justified: indeed, americium was also found in the studied samples!
True, the content of curium in nature is vanishingly small: in the studied samples it did not exceed one hundred millionth fractions of a percent. But the fact that, in addition to plutonium, sauranium elements, up to and including curium, are created not only in laboratories, but also in the depths of planets and stars, has been proven.
N. Ivanov, A. Livanov, V. Fedchenko
|
 Transuranic elements are the brainchild of modern technology. Laboratories with sophisticated equipment, nuclear reactors - these are the "deposits" from which, at the cost of huge energy expenditures, are now receiving insignificant quantities of elements that are not found in nature.
Transuranic elements are the brainchild of modern technology. Laboratories with sophisticated equipment, nuclear reactors - these are the "deposits" from which, at the cost of huge energy expenditures, are now receiving insignificant quantities of elements that are not found in nature. The study of strange samples has shown that they have an excess of uranium-235 compared to the theoretically calculated amount. But uranium-235 is the final decay product of the sauranium element curium obtained in the laboratory. If this is so, then in nature there is not a short-lived laboratory curium, but some of its long-lived isotope.
The study of strange samples has shown that they have an excess of uranium-235 compared to the theoretically calculated amount. But uranium-235 is the final decay product of the sauranium element curium obtained in the laboratory. If this is so, then in nature there is not a short-lived laboratory curium, but some of its long-lived isotope.